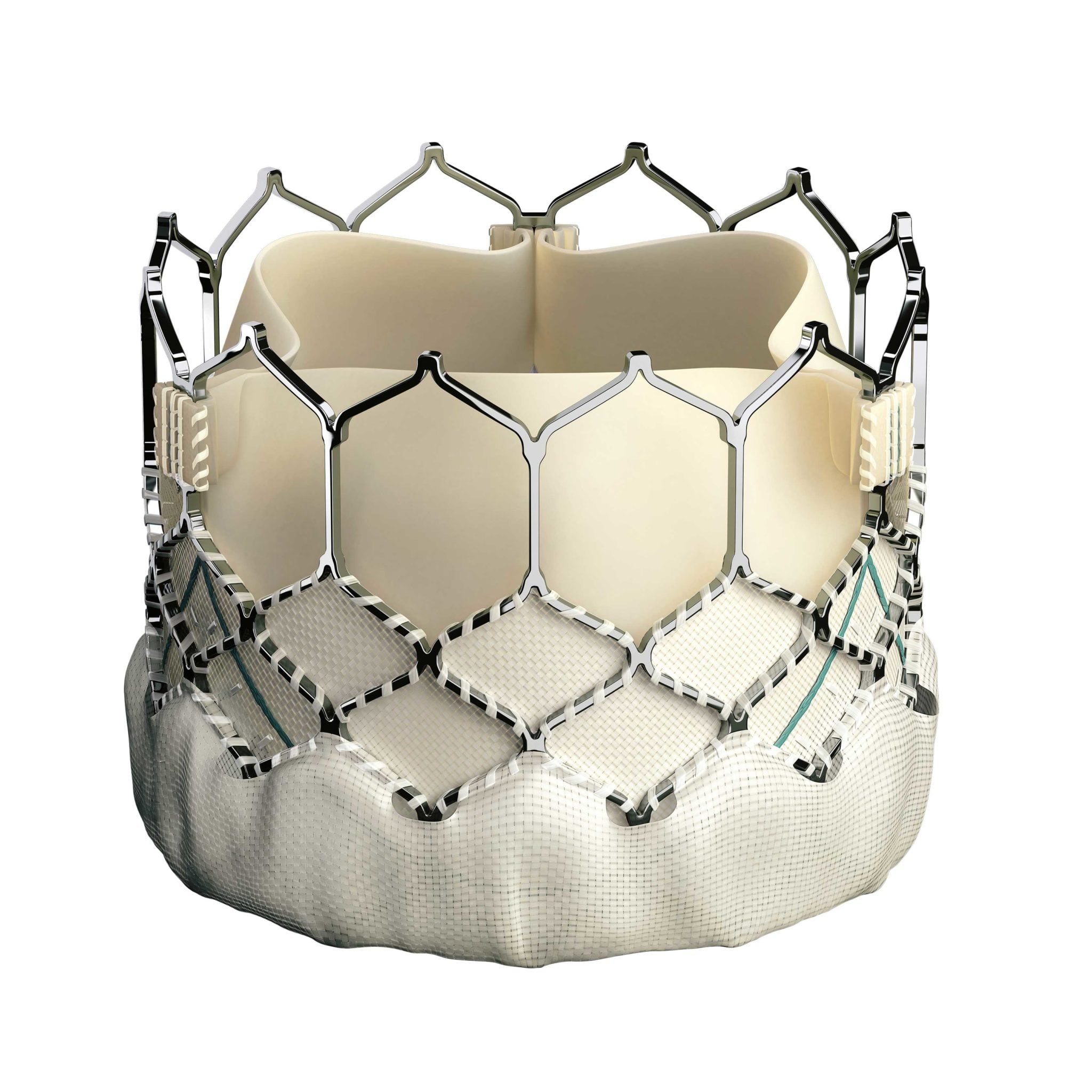
The expanded use of a heart-valve replacement technology for patients too ill for open-heart surgery, approved only in June by the Food and Drug Administration, was adopted quickly by doctors at the Heart & Vascular Institute.
Transcatheter Mitral Valve Replacement, or TMVR, allows doctors to replace a malfunctioning mitral valve — either the patient’s own or bioprosthetic valve from a previous surgery — using a small tube called a catheter inserted into a large vein in the groin instead of conventional open-heart surgery. The FDA’s approval applies specifically to the Sapien 3 Transcatheter Heart Valve from Edwards Lifesciences.
“Approval is limited to patients who are deemed a high risk for repeat open-heart surgery,” says Dr. Raymond McKay, one of the doctors who performed the first TMVR procedure at the Heart & Vascular Institute in August, “and only for patients who have already undergone previous surgical mitral valve replacement.”
The mitral valve, two flaps between the left atrium and left ventricle, controls blood flow between the two chambers. It’s a one-way valve, allowing blood from the left atrium into the left ventricle while preventing the reverse flow into the left ventricle. Mitral valve regurgitation, when the valve doesn’t close properly and allows backward blood flow, can cause shortness of breath, fatigue, swollen ankles or feet and heart palpitations.
TMVR uses the same technology as the Transcatheter Aortic Valve Replacement, or TAVR, approved by the FDA in November 2011 for use in inoperable patients and in October 2012 for use in high-risk patients. The procedure, which places a new aortic valve within the existing valve, is also being used now in moderate-risk cases.
Some hospitals use what’s known as off-label TAVR, not approved by FDA — about 10 percent of all TAVR procedures in the United States. These procedures were associated with higher in-hospital 30-day and one-year mortality rates, though the one-year mortality rate was similar to the FDA-approved TAVR, according to the Transcatheter Valve Therapy Registry.